CityU neuroscientists discover a new drug candidate for treating epilepsy
Temporal lobe epilepsy (TLE) is one of the most common types of epilepsy worldwide. Although symptomatic medications are available, one-third of TLE patients remain unresponsive to current treatment, so new drug targets are critically needed. A research team co-led by a City University of Hong Kong (CityU) neuroscientist recently identified and developed a new drug candidate that has potential for effectively treating TLE by suppressing neuroinflammation.
Epilepsy is one of the most prevalent chronic brain disorders and is characterised by recurrent and spontaneous seizures. Most anti-epileptic drugs that are currently available target neurons and synapses in the brain. They are effective in changing neural circuits and synapses, but this treatment overlooks another important pathology: neuroinflammation.
(source: Juan A. Orellana, Synaptic Functions of Astroglial Hemichannels, DOI: 10.5772/intechopen.87142. https://www.intechopen.com/chapters/67921)
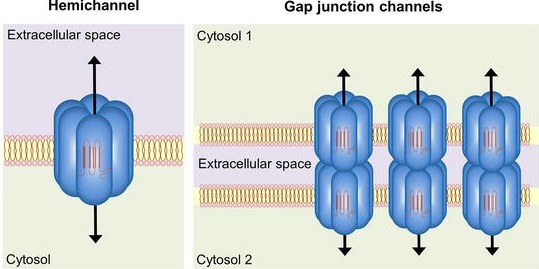
Neuroinflammation is caused by the abnormal functioning of reactive glial cells, such as astrocytes and microglia, causing an immune reaction in the brain. Accumulating evidence points to a key role of connexin-based gap junctions and hemichannels in brain glial cells in TLE. A hemichannel is a channel or pathway formed by the assembly of six proteins, which permits small molecules such as glutamate to be released from astrocytes and microglia to extracellular space. A gap junction is formed when the hemichannels of two adjacent cells dock with each other, as shown in Figure 1. But inhibiting both gap junctions and hemichannels can lead to undesirable side effects because the former coordinate physiological functions of cell assemblies. Therefore, scientists need to find a way to block only connexin hemichannels to effectively reduce neuroinflammation with fewer side effects.
A research team co-led by Dr Geoffrey Lau Chun-yue, Assistant Professor in the Department of Neuroscience, identified a new, small organic molecule called D4, which selectively blocks connexin hemichannels, but not gap junctions. The team investigated its effect in treating TLE using a mouse model. The findings suggest that D4 strongly suppresses the TLE-induced neuroinflammation, curbs TLE seizures, and increases the animal’s survival rate.
The findings were published in the international scientific journal Proceedings of the National Academy of Sciences of the United States of America (PNAS) under the title “Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy”.
New drug D4 suppresses neuroinflammation
“These are very exciting and encouraging results for translational research in epilepsy,” said Dr Lau. “We have found a very promising new drug candidate for treating epilepsy that works through a new mechanism – blocking connexin hemichannels. Our findings also highlight the important involvement of neuroinflammation in neurological disorders such as epilepsy.”
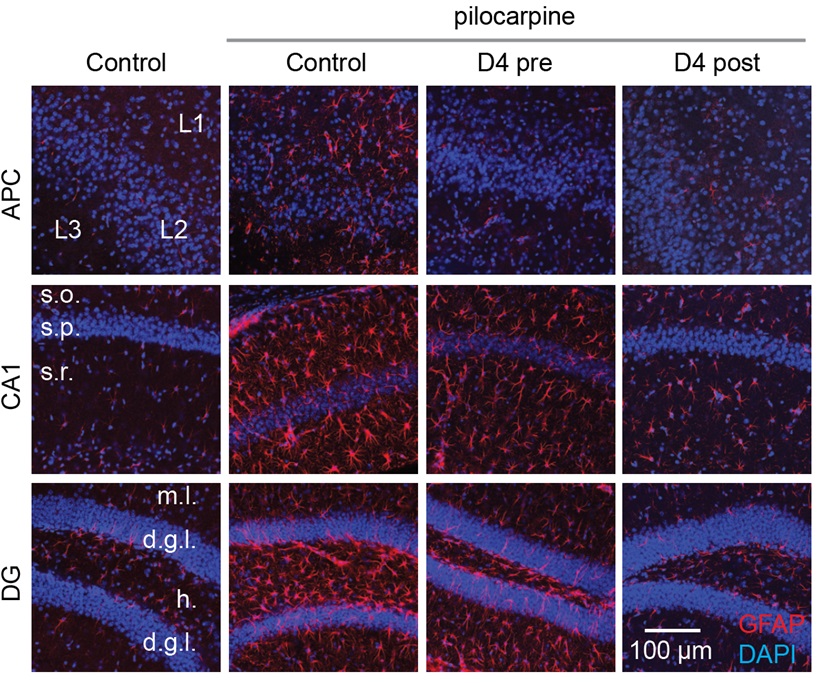
The new drug, D4, targets a new class of ion channels, the connexin hemichannels in the glial cells. Glial cells include astrocytes and microglia and are important for modulating neurotransmission. Excessive glutamate and other molecules can leak out from reactive glia via hemichannels to the extracellular environment, altering synapses, enhancing neuroinflammation and exacerbating seizures. By specifically blocking connexin hemichannels using D4, Dr Lau’s team can directly target neuroinflammation caused by astrocytes and microglia.
The research adopted the pilocarpine model of epilepsy in mice, a well-known model to produce phenotypes that resemble human TLE. Pilocarpine was injected into mice intraperitoneally to induce seizures. The administration of one dose of D4 orally before inducing seizures effectively reduced neuroinflammation and altered synaptic inhibition, which increased the animal’s survival rate. For treatment after induced seizures, a single dose of D4 had a prolonged effect on suppressing the activation of astrocytes and microglia. This suggests that D4 strongly alleviates neuroinflammation and has a long-term effect.
A single dose offers long-term benefits
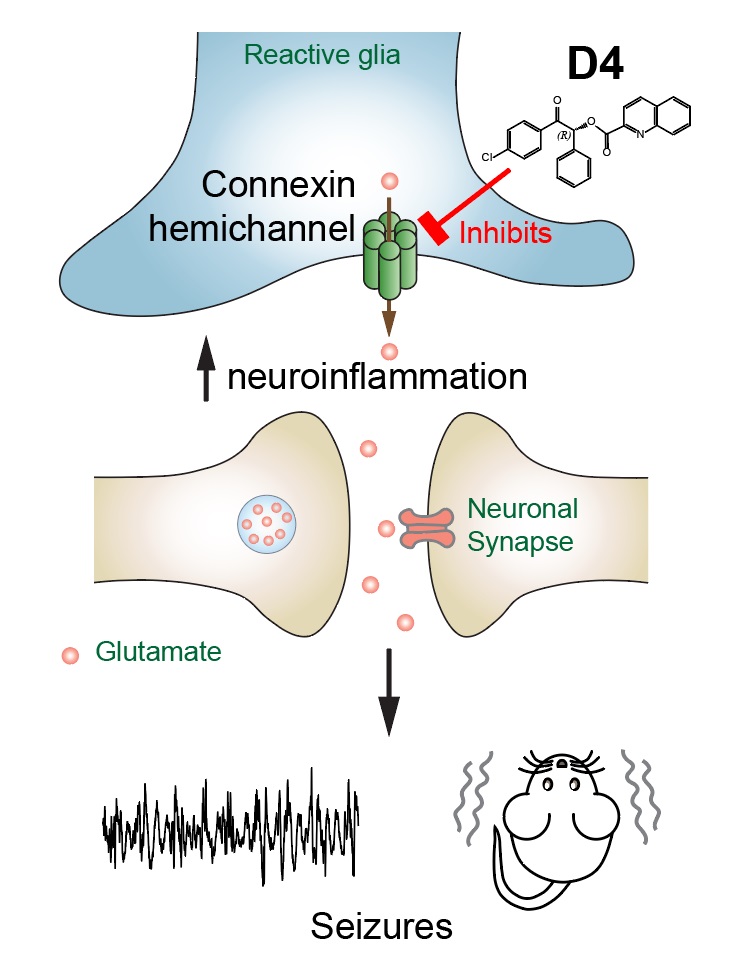
Photo source︰© Dr Geoffrey Lau
Results from both pre- and post-treatment indicate that targeting connexin hemichannels by D4 is an effective and promising strategy for treating epilepsy in which neuroinflammation plays a critical role. The drug can be taken orally to effectively get into the mouse brain to reduce the harmful effects of neuroinflammation. A single dose provides strong protection against future seizures. “We hope that this will ultimately result in new and better treatment options for epileptic patients,” said Dr Lau. The team will continue to work on the astrocytic mechanisms of epilepsy and identifying more new therapeutic targets.
© City University of Hong Kong
The first author of the paper is Dr Guo Anni, CityU PhD graduate and a postdoctoral fellow in Dr Lau’s laboratory. Corresponding co-authors include Dr Lau and Professor Juan C Saez, from the University of Valparaíso, in Chile. Dr Lau’s PhD student, Zhang Huiqi, and research assistant, Li Huanhuan, also participated in the research. The research was funded by CityU, the Hong Kong Research Grants Council, InnoHK and the Shenzhen General Basic Research Program.