CityU materials scientists find a new way to create thermally stable high-entropy alloys
Nanoparticles have been used to develop high-strength materials for structural applications. But these nanoparticles are often thermally unstable, leading to rapid coarsening in a high-temperature environment. The latest research led by materials scientists at City University of Hong Kong (CityU) found that tailoring the concentration of cobalt in high entropy alloys (also called chemically complex alloys) can prevent nanoparticles from rapid coarsening at high temperatures. This novel stabilising strategy opens a new pathway to design novel thermally stable chemically complex alloys for various engineering fields in the future.
Nanoparticle-strengthening technology – strengthening alloys by adding nanoparticles in the alloying process – is regarded as a powerful strategy to create materials with unique structural and functional properties. This has been widely applied to innovate high-strength materials, like advanced aluminium alloys, steels and superalloys. But these fine particles at the nanoscale have poor thermal stability and are prone to rapid coarsening at high temperatures, which dramatically decreases the load-carrying capacity of the host materials and consequently leads to their fracture or other catastrophic failures.
To overcome this obstacle, a research team co-led by CityU materials scientists recently revealed that tailoring the cobalt concentration can controllably govern the “sluggish lattice diffusion” effect of high-entropy alloys in a quantitative manner, substantially preventing nanoparticles from rapid coarsening at high temperatures up to 1,000°C.
“Our findings pave a highly effective pathway for the well-targeted design of high-performance alloys with excellent thermal and mechanical properties for high-temperature structural applications,” said Dr Yang Tao in the Department of Materials Science and Engineering (MSE) at CityU, who led the study. The research findings were published in the scientific journal Nature Communications under the title “Achieving thermally stable nanoparticles in chemically complex alloys via controllable sluggish lattice diffusion”.
The sluggish lattice diffusion effect means the diffusion of individual elements in alloys with higher configurational entropy is slower than those with lower configurational entropy. This can potentially endow several high-entropy alloys with remarkable thermal stability. But the underlying mechanism of the sluggish lattice diffusion effect is still unknown.
© Xiao, B. et al. (Source: https://www.nature.com/articles/s41467-022-32620-6)
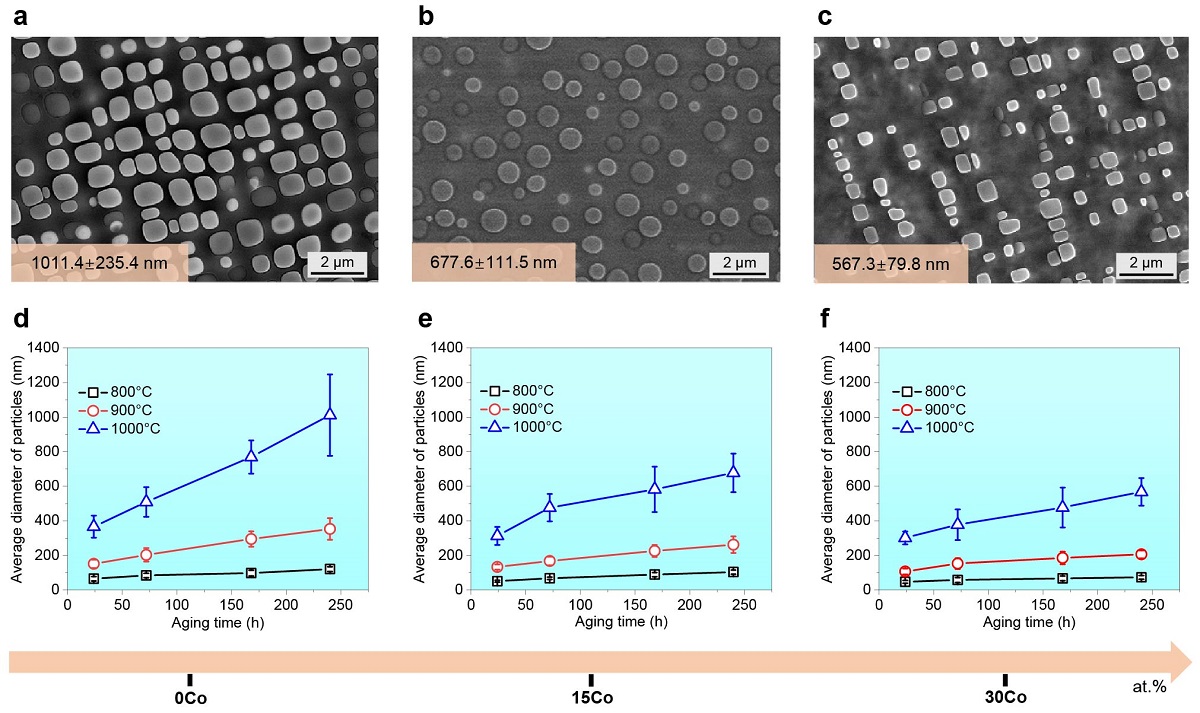
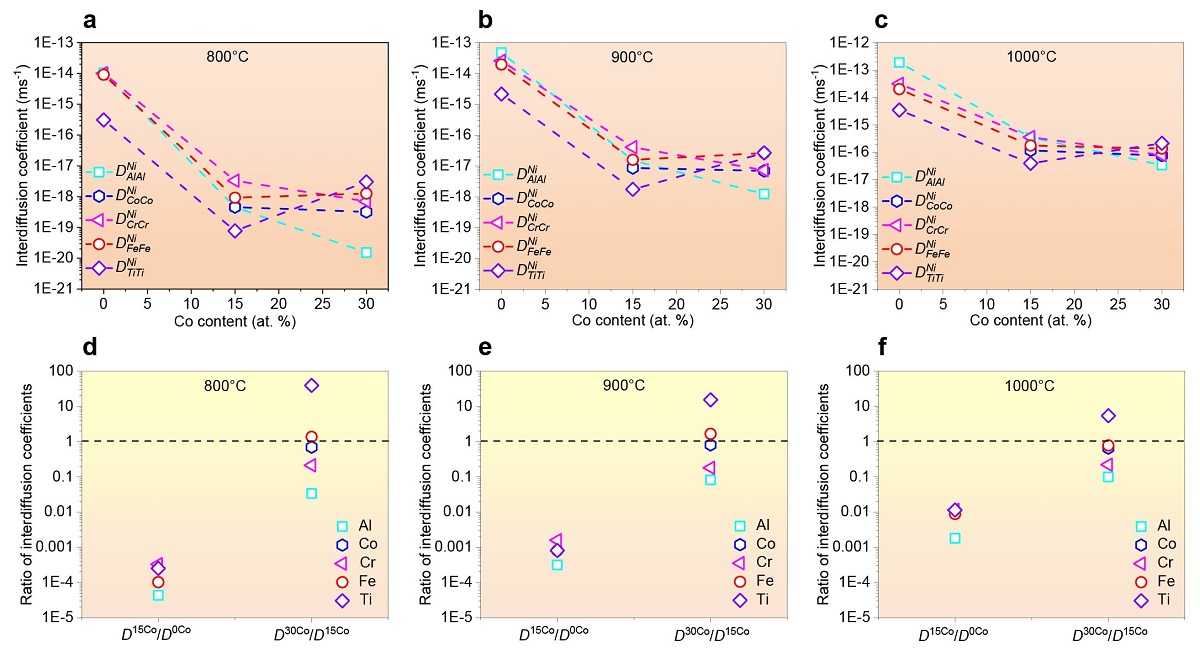
In this study, through a combination of various complementary experimental techniques and theoretical simulations, the research team found that cobalt can effectively trigger a unique sluggish lattice diffusion effect in the nickel-cobalt-iron-chromium-aluminium-titanium (NiCoFeCrAlTi) alloy system by decreasing the interdiffusion coefficient (a parameter to describe atom mobility in a material) of other elements. They found that increased concentrations of cobalt can substantially reduce the average particle size and further improve the thermal stability of these nanoparticles.
© Xiao, B. et al (Source: https://www.nature.com/articles/s41467-022-32620-6)
Moreover, tailoring the concentration of cobalt led to a significant reduction in the interdiffusion coefficients of all the main constitutions of high-entropy alloys, especially aluminium, at 800°C.
The controllable sluggish lattice diffusion strategy developed by the research team can achieve ultra-stable nanostructures in high-entropy alloy systems at 800 to 1,000°C.
“We discovered a novel nanoparticle stabilising mechanism that is distinctively different from the conventional wisdom that nanoparticle stabilisation is achieved by adding refractory elements, like rhenium,” explained Dr Yang.
“This new strategy can further guide the development of novel chemically complex alloys with superior microstructure stability and be potentially applied to other metallic alloys. This paves the way to develop strong, next-generation, high-entropy alloys that can be used in an extreme, high-temperature environment in various engineering fields, such as aerospace, automotive design and nuclear engineering,” he said.

The first author of the research is Dr Xiao Bo. The corresponding author is Dr Yang, from MSE, and Professor Kai Jijung, from MNE. Other collaborators include Professor Liu Chain-tsuan, Dr Luan Junhua and Dr Zhao Shijun, from CityU, and researchers from Central South University, Harbin Institute of Technology (Shenzhen), and Tianjin University.
The funding sources for the research were CityU, the Hong Kong Research Grant Council, the National Natural Science Foundation of China, and the Guangdong Basic and Applied Basic Research Foundation.