Novel perovskite material with a special structure can generate hydrogen without cocatalyst
Hybrid organic–inorganic perovskite can be used in photocatalytic hydrogen generation. However, the traditional lead-based perovskite contains the toxic metal lead, therefore its application is hindered. Recently, a joint research team led by a scientist from City University of Hong Kong (CityU) has achieved a breakthrough in fabricating a novel lead-free perovskite material with a special structure. Without using cocatalyst, this material showed a three-fold improvement in the efficiency of solar-to-hydrogen energy conversion. Their findings can not only lower the production cost but also provide new insights into the development of solar-to-fuel materials for photocatalytic applications in the emerging field of hydrogen technology.
The research team is led by Dr Sam Hsu Hsien-yi, Assistant Professor of the School of Energy and Environment (SEE) as well as the Department of Materials Science and Engineering at CityU. The research achievement has been published in the scientific journal Advanced Functional Materials, titled “In Situ Formation of Bismuth‐Based Perovskite Heterostructures for High‐Performance Cocatalyst‐Free Photocatalytic Hydrogen Evolution.” It has been featured on the inside front cover of the journal.
Lowers the production cost as cocatalyst no longer required
Over the years, Dr Hsu has researched on energy engineering as well as the design and synthesis of materials like perovskite. “Without using noble metal such as platinum as a cocatalyst, the photocatalytic performance of hybrid perovskites is unsatisfactory. However, the noble metal is very expensive, and this creates a dilemma,” explained Dr Hsu.
The cocatalyst is a substance that facilitates the function of catalyst. It can provide trapping sites for the photogenerated charges and promote the charge separation.
With over a year of study, Dr Hsu’s team came up with a solution. They became the first team to produce a heterojunction of lead-free bismuth-based perovskite material that could drive photocatalytic hydrogen production without cocatalyst. They adopted the solvent-engineering technique by adding a solvent named dimethylformamide (DMF) to the isopropanol (IPA) solution. In this way, they successfully fabricated a novel type of lead-free bismuth‐based hybrid organic-inorganic perovskite material with heterostructures. This perovskite can generate hydrogen steadily from hydrogen iodide with irradiation by the visible light source.
Surprising structure found in the new material
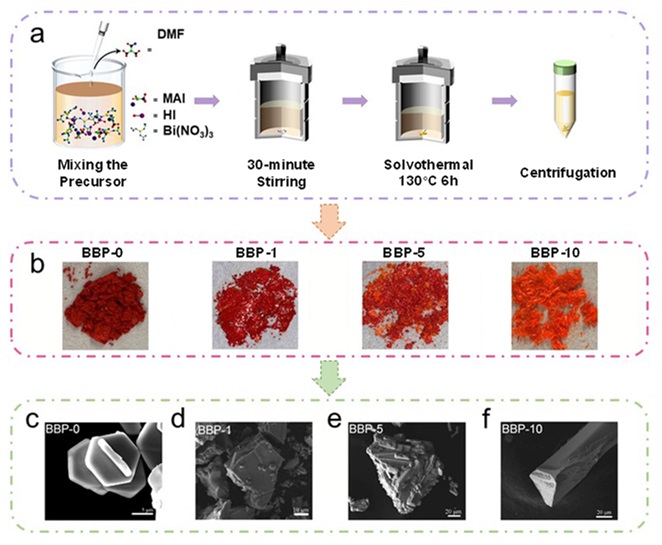
The team observed the structure of the novel material by microscope and discovered that adding DMF would cause a chemical reaction on this bismuth‐based perovskite material, namely methylammonium bismuth iodide (MA3Bi2I9), to form tri(dimethylammonium) hexa-iodobismuthate (DMA3BiI6). When the DMF concentration reached 5%, the co-existing MA3Bi2I9 and DMA3BiI6 would form heterojunctions, an interface that occurs between two layers of different semiconductors.
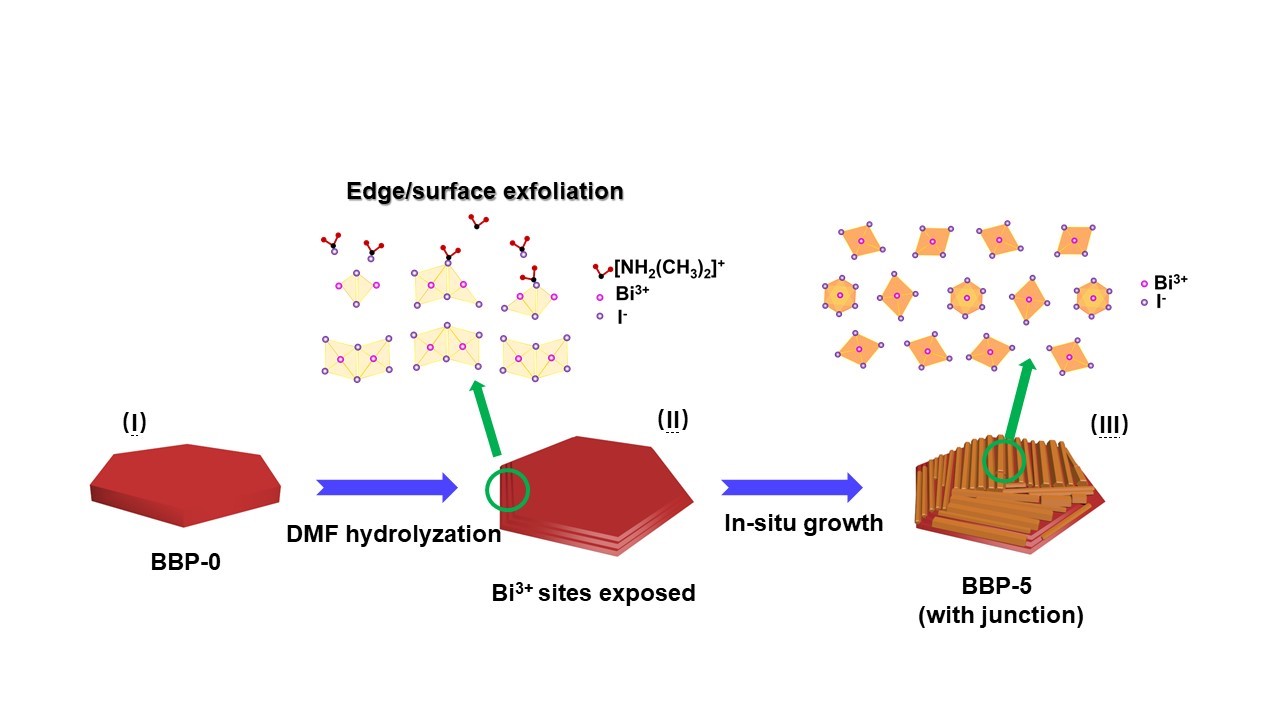
“DMF would undergo hydrolysis and then react with the bismuth ions in the perovskite, resulting in the in‐situ formation of the heterostructures. We found no heterojunctions when 1% or 10% DMF is added. But heterojunctions were formed when the DMF concentration is 5%. This is indeed a surprising and interesting finding,” said Dr Hsu.

Then what are the advantages of having heterojunctions? They enable energy band alignment, which means the energy band of the two materials come together. As a result, the efficiency of charge separation is enhanced. Charge separation means separating a pair of an electron and hole so that they can move in different directions respectively. After separation, electrons can be transferred to the material interface and then react with other substances.
During the process of hydrogen iodide splitting, charge separation was enhanced by the perovskite heterojunctions so that the electrons and holes could “move” separately. This enabled the electrons to react with hydrogen ions to generate hydrogen. Moreover, the photoluminescence lifetime of the perovskite was also significantly enhanced due to the energy band alignment of the heterojunctions. Therefore, electrons had a higher chance to react with hydrogen ions, thus the efficiency of hydrogen evolution was enhanced.
Impressive hydrogen evolution rate achieved without using cocatalyst
Experiment results showed that this novel type of lead-free bismuth‐based perovskite material achieved the hydrogen evolution rate of 198.4 µmol h−1 g−1 without the addition of platinum as the cocatalyst under visible‐light illumination. “This result is even higher than similar perovskite material without DMF but using platinum as cocatalyst. And it is proven in the experiments that the solar‐driven photocatalytic activity of our perovskite is stable under continuous illumination for 100 hours,” Dr Hsu emphasised.
Their next step is to enhance hydrogen production capacity. Dr Hsu believed that in the long run, hydrogen would become one of the major energy sources. He hoped that this research would help to harvest solar energy in response to the global challenge of the energy crisis.
Dr Hsu is the sole corresponding author of the paper. The first author is his PhD student at SEE, Tang Yunqi. Other CityU co-authors are Professor Wang Zuankai from the Department of Mechanical Engineering, Stanley Mak Chun-hong, and Liu Rugeng, both PhD students from SEE. Other research team members come from Fudan University, Tsinghua Shenzhen International Graduate School, and the University of Rennes 1.

The research was supported by CityU, the National Natural Science Foundation of China, Shenzhen Science and Technology Innovation Commission, the Research Grants Council of Hong Kong, and RGC-PHC France/Hong Kong PROCORE program.
DOI number: 10.1002/adfm.202006919
This research has been featured on the inside front cover of the journal Advanced Functional Materials: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202070343
Related story: CityU scientist's technology for generating renewable energy awarded APEC Prize