A novel method for identifying stomach cancer cells within 5 minutes
A novel method developed by a joint research team led by a scientist from City University of Hong Kong (CityU) can identify gastric cancer cells within minutes. This fast method with high sensitivity brings hope in speeding up the diagnosis of gastric cancer.
Led by Professor Li Wen Jung, Chair Professor of Biomedical Engineering from CityU’s Department of Mechanical Engineering and Associate Provost (Institutional Initiatives), the research team published their findings recently in the scientific journal Science Advances, titled “Detection and isolation of free cancer cells from ascites and peritoneal lavages using optically induced electrokinetics (OEK)”.
“Our research aims to reduce the number of deaths due to gastric cancer, one of the leading causes of cancer deaths worldwide,” said Professor Li.
Around 800,000 deaths per year worldwide are due to gastric cancer, the third-highest rate among cancer deaths. Since early-stage gastric cancer is commonly asymptomatic, most of the gastric cancer patients are diagnosed as the advanced stage. Among the advanced gastric cancer cases, peritoneal metastases is one of the most common modes of cancer dissemination. But an early diagnosis of peritoneal metastasis is difficult because current approaches lack sensitivity.
In clinical practice, cytological examinations of ascites (abnormal build-up of fluid in the abdomen) and peritoneal lavages (an invasive test to obtain specimens by irrigation of the peritoneal cavity) are performed to detect the cancer cells. However, these approaches are not sensitive enough to identify the few cancer cells among a large number of cells. The process also involves cell staining, which is time-consuming and does not provide live cells for further examination.
Apply the OEK method to isolate cancer cells
Professor Li has been exploring the use of optically induced electrokinetics (OEK) as an alternative for the detection and isolation of cancer cells for years. “This is the first time that the OEK method was successfully applied to the rapid separation of living gastric cancer cells from patients’ ascites samples,” said Professor Li.
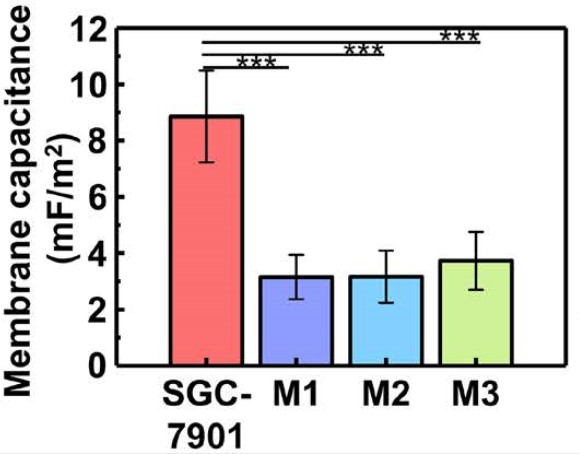
The OEK method is a new technique integrated with “lab-on-a-chip” systems which can offer researchers opportunities to manipulate objects within a micro- and nanoscale bioengineering environment. The OEK mechanism relies on the difference in size between the gastric cancer cells and peritoneal lavage cells. With the larger cell size, the cancer cells possess higher cell membrane capacitances than the peritoneal lavage cells.
In their experiments, the researchers first placed the patients’ ascites in the OEK microfluidic chip. By projecting movable light patterns on the chip, the conductivity of the illuminated area increased. Hence the moving light patterns effectively became dynamic (meaning movable in a controllable way) electrodes on the chip’s surface. Then, by applying an AC voltage on the chip, controllable and movable non-uniform electric fields could be generated in the solution. Hence the cells could be manipulated by the OEK force exerted on them.
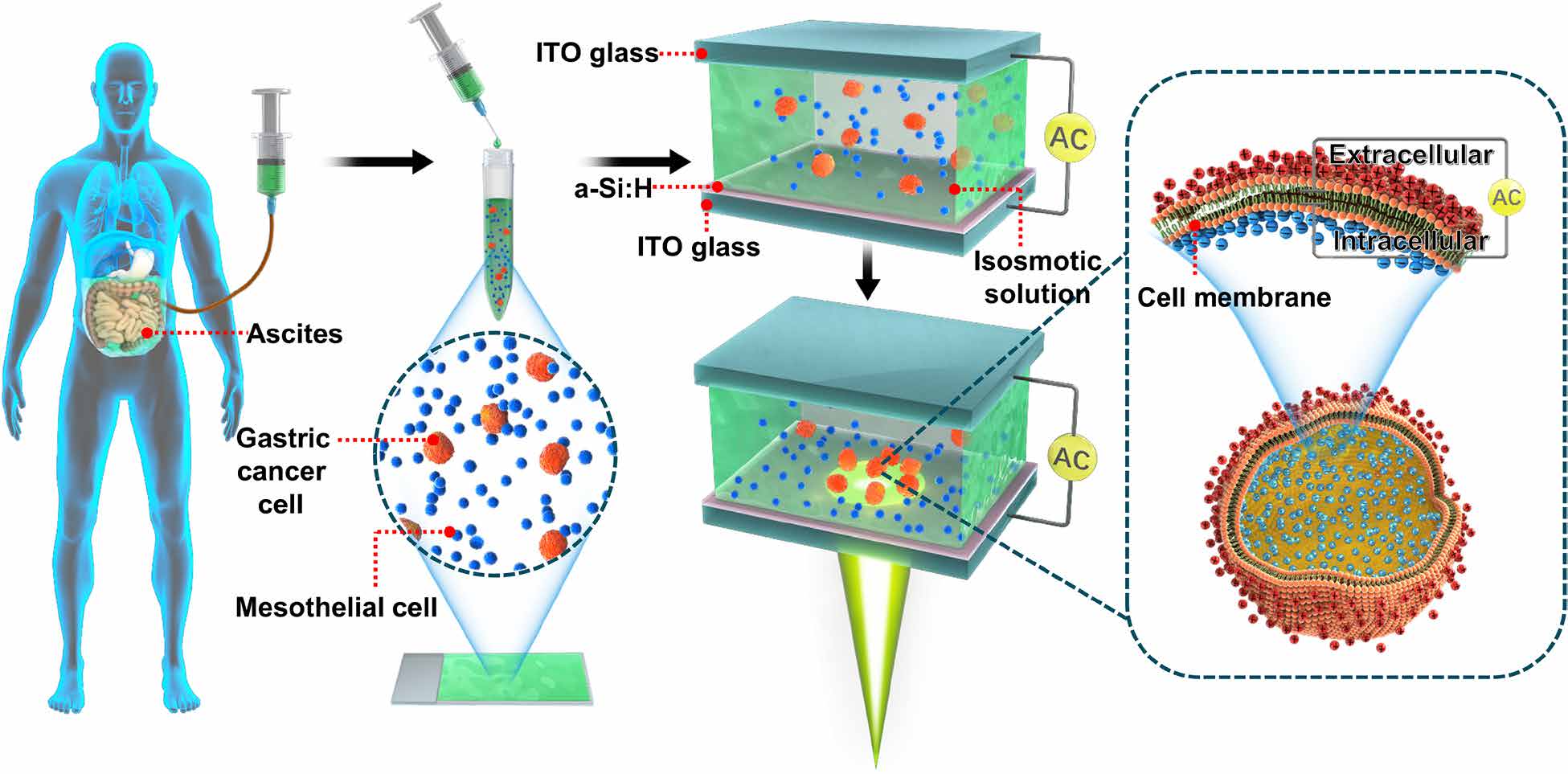
Dragging the cancer cells with a “magnet”
“Due to the difference in cell membrane capacitances, the cancer cells were attracted to the light pattern like a magnet, while the peritoneal lavage cells were repelled from it,” explained Professor Li. Then the researchers could “drag” the cancer cells by moving the light pattern towards the plastic hose connected with the chip for collection, successfully separating the cancers cells from the ascites.
This video shows that cancer cells are attracted to the light pattern and separated from other cells. (Video source: DOI: 10.1126/sciadv.aba9628)

The new method is appealing because it is quick and label-free. In fact, within just five minutes, it can separate gastric cancer cells on the OEK microfluidic chip. “It usually takes 6 or 7 hours for the traditional method to identify the cancer cells. The OEK method is much faster,” Professor Li said.
Faster and more sensitive testing
Moreover, the OEK method is more sensitive. “Using this technique, we have been able to separate gastric cancer cells from other cells in six patients’ ascites sample with a success rate of 71%,” he added. In their sensitivity testing, they successfully separated cancer cells from peritoneal lavage cells up to a ratio of 1:1000.
“The study has benefited from working with doctors and patients at First Hospital of China Medical University in Shenyang where medical staff have been impressed by the results. The researchers from the hospital would like to continue this OEK-based research with SIA and CityU to eventually use it as a clinical process,” Professor Li said.
Suitable for patients with various treatment histories

By using samples from patients with different treatment backgrounds, their experimental results showed that the system is suitable for ascites cells from various patients and treatment histories, which demonstrates a bright prospect for the wide clinical usage in the future. They also measured the cell membrane capacitances, which may be used as a biomarker for gastric cancer cells. “We hope our research will speed up the diagnosis of gastric cancer and save lives.”
Professor Li is also an Affiliated Professor of the Shenyang Institute of Automation (SIA) of the Chinese Academy of Sciences (CAS). He first set up the OEK system in CityU some years ago. With the support of the CAS-Croucher Funding Scheme, he later replicated the system at the Joint Laboratory co-established by CityU and the SIA in Shenyang, where the experiments of this study were done.
Professor Li, Professor Wang Zhenning of the First Hospital of China Medical University in Shenyang, and Dr Yu Haibo from the SIA are the corresponding authors of the paper. The co-first authors are Zhang Yuzhao, Professor Li’s PhD student at SIA, and Zhao Junhua from the First Hospital of China Medical University. Other researchers are from institutions including Shenyang Jianzhu University and National Tsing Hua University.
The funding support for the study included the National Natural Science Foundation of China, the National Key R&D Programme of China, and the Hong Kong Research Grants Council.
DOI number: 10.1126/sciadv.aba9628