Genetically modified neural stem cells developed by CityU and HKUMed researchers show promising therapeutic potential for spinal cord injury
A research team co-led by City University of Hong Kong (CityU) and The University of Hong Kong (HKU) has recently made a significant advancement in spinal cord injury treatment by using genetically modified human neural stem cells (hNSCs). They found that specifically modulating a gene expression to a certain level in hNSCs can effectively promote the reconstruction of damaged neural circuits and restore locomotor functions, offering great potential for new therapeutic opportunities for patients with spinal cord injury.
Traumatic spinal cord injury is a devastating condition that commonly results from accidents such as falls, car crashes or sport-related injuries. Spinal neurons with long axons play critical roles in transmitting signals between the brain and the rest of the body, controlling our movement and sensory perception. Spinal cord injury causes irreversible damage to neurons and axons, which significantly interrupts signal transmission, hence leading to defective locomotion and somatosensory functions.

“Currently, there are no effective clinical management or treatment regimens for patients with spinal cord injury, often leaving them with lifelong disabilities,” explained Professor Jessica Liu Aijia, Assistant Professor in the Department of Neuroscience at CityU and the co-leader of the research. “While recent progress has been made in promoting spinal cord regeneration through transplantation of hNSCs derived from human induced pluripotent stem cells, the degree of functional recovery obtained has been modest. This is largely due to the hostile micro-environment around the lesion site, such as the formation of barrier-like structures called astroglial scars and the lack of neurotrophic factors in adults for neuronal differentiation. These factors hinder functional neuronal regeneration, resulting in prolonged repairing and limited functional recovery.”
A gene – SOX9 was reported with a high-level expression at the injury site in previous studies, and the gene itself is the leading cause of the glial scars’ formation and the hindrance of neuronal survival and differentiation. To overcome the adverse effects of the post-injury micro-environment, the joint-research team engineered the transplanted neural stem cells with a graded reduction of SOX9 by approximately 50%.
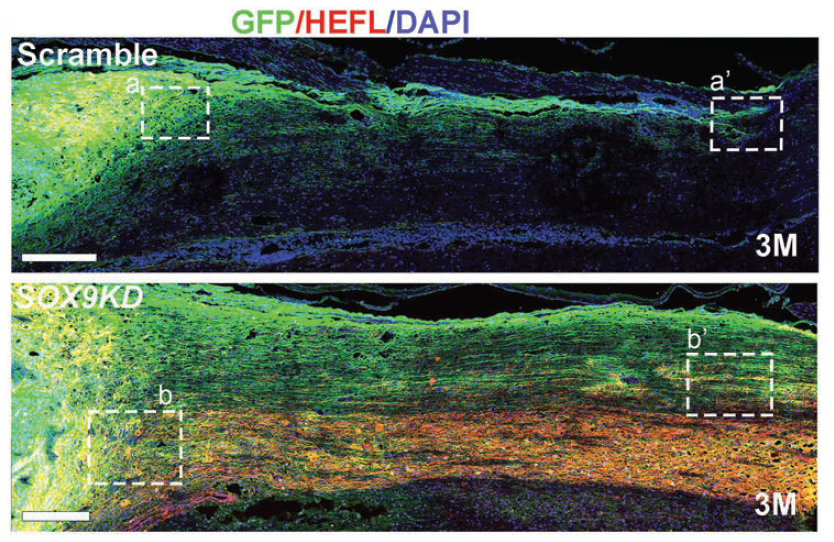
The genetically modified hNSCs expressing half-dose of SOX9 resulted in robust neuronal differentiation and maturation (a process by which immature cells in the nervous system become specialised nerve cells) after transplantation in a hostile microenvironment, promoting neural circuits reconstruction in the spinal cord within a shorter period of time. It also remarkably reduced glial scar accumulation, facilitating long-distance axon outgrowth. The team recorded a large number of axons extending more than 35mm at 3 months post-graft, while relatively less in number with shorter extensions to 25mm with non-modified neural stem cells.
To further explore the therapeutic effects of the modified hNSCs in treating spinal cord injury, the research team used a severe spinal cord injury rat model to evaluate the locomotion recovery after transplantation, including grid walking and consecutive walk. The former recorded limb coordination, such as the capability to grasp a grid rung with correct placement, and the latter recorded stepping patterns to demonstrate their gait and fingertip motor ability.
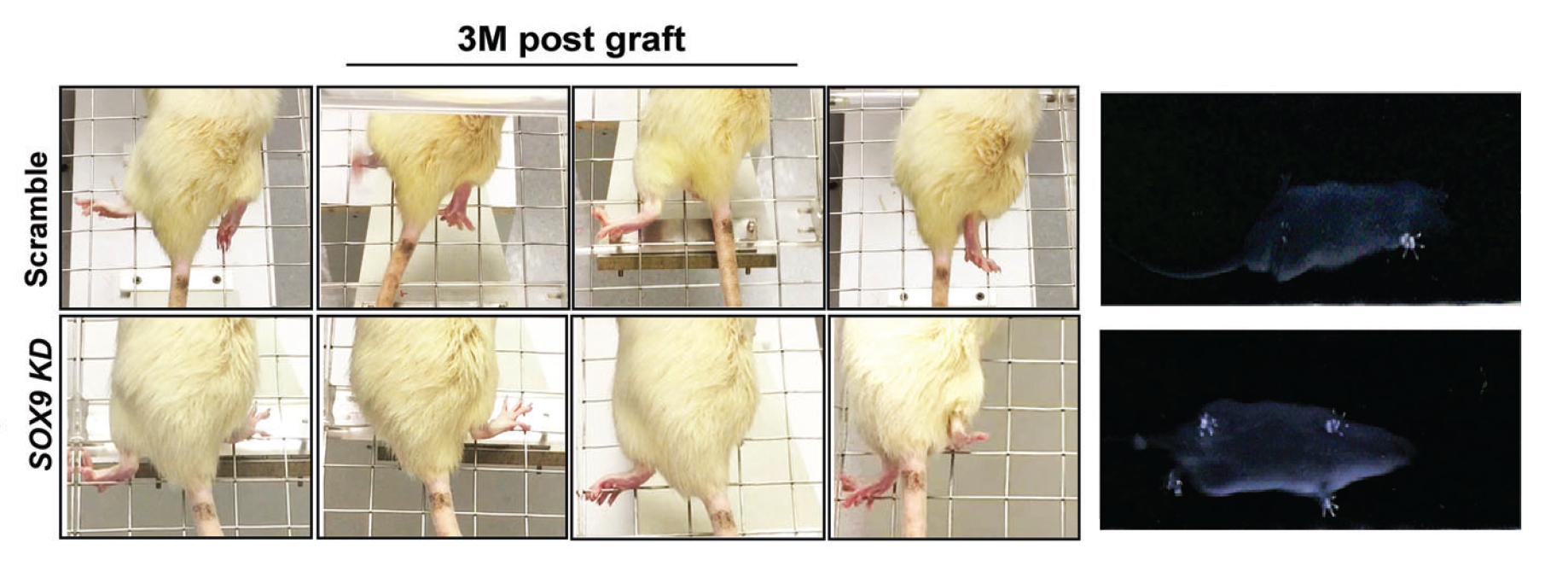
Compared to rats’ graft with non-modified hNSCs, rats' graft with SOX9 gene-modified hNSCs performed much better in placing their affected hind paws on the grid, with fewer misdirected steps after 10 weeks post-injury. In addition, these treated rats demonstrated a good gait with clear paw position and toe movement when walking across a metre-long narrow corridor.
“Our findings reveal a new treatment direction by using a genetically modified strategy to alter the grafts’ response to the deleterious microenvironment in vivo after injury, improving cell tolerance to the niche and self-differentiation potential. This brings a new treatment direction for repairing damaged spinal cord,” said Professor Liu.
“This genetic modification of hNSCs, particularly those derived from patient-specific human-induced pluripotent stem cells, which can be generated from a patient's skin or blood cells, eliminates ethical concerns in using embryonic stem cells and minimises the risk of rejection by the immune system. It provides a more effective autologous stem cell therapy for severe traumatic spinal cord injury,” she added.
The findings were published in the scientific journal Advanced Science under the title “Transplanting Human Neural Stem Cells with ≈50% Reduction of SOX9 Gene Dosage Promotes Tissue Repair and Functional Recovery from Severe Spinal Cord Injury”.
The paper’s first co-authors and the corresponding authors are Professor Liu and Dr Martin Cheung Chi-hang, Associate Professor in the School of Biomedical Sciences, Li Ka Shing Faculty of Medicine at HKU (HKUMed). The other collaborators include Professor Chan Ying-shing of HKUMed and researchers at CityU and HKUMed. The research has been funded by Hong Kong Research Grants Council.