Regulatory mechanisms of virulence of a plant bacterial pathogen unveiled facilitating the development of new anti-bacterial drugs
With an increasing global population, we often heard warnings about the possibility of a food supply shortage. In addition to climate change and the reduction of agricultural land, the world's food security is also threatened by bacterial diseases of plants. A joint research team led by scientists from City University of Hong Kong (CityU) has previously identified a pathogen’s transcription factors, the key proteins that are virulence-related. Recently, the team has further revealed the regulatory mechanisms of virulence of that pathogen, facilitating the development of new anti-bacterial drugs for prevention and treatment. Their findings could also promote future studies on that pathogen and the transcription factors of other pathogens.
The research team was co-led by Dr Deng Xin, Associate Professor in the Department of Biomedical Sciences (BMS) at CityU, together with Dr Yan Jian, Assistant Professor in the same department. Their findings have been published in the scientific journal Nature Communications, titled “A compendium of DNA-binding specificities of transcription factors in Pseudomonas syringae”.
Food security threatened by bacterial diseases of plants
Pseudomonas syringae (P. syringae) is a common type of Gram-negative bacterium. It is a group of microorganisms widely found in the natural environment like soil, air and leaves’ surfaces. This plant pathogen can infect a wide variety of fruits, vegetables, and plants, including major crops for humans such as rice and soybean, and tomato and pea.
“P. syringae is widely recognised as the most prominent plant bacterial pathogen, which causes crop failure and threatens the world's food security. Although it causes huge economic loss in the worldwide agriculture sector every year, currently there are only limited ways to fight against it,” said Dr Deng.
To develop novel drugs that are more efficient, scientists need to understand the pathogenic mechanisms of P. syringae.Prior to this research, Dr Deng’s research team has already revealed that the virulence of P. syringae is under the control of a large group of transcription factors (TFs). TFs are proteins which can bind with specific DNA sequences to regulate gene expression by turning the specific genes “on” and “off”, generally a key determinant in whether the gene functions at a given time.
“Although previously we have discovered the TFs, the overall regulatory mechanisms of virulence remain unexplored in P. syringae. For example, the TFs are to be bound with which specific DNA sequences so that P. syringae would become pathogenic? Not until we have a complete picture of binding profiles of all TFs and their precise targets in the genome, it is difficult to prevent large scale disease outbreak caused by P. syringae completely,” Dr Deng elaborated.
Unveiling the regulatory mechanisms of virulence
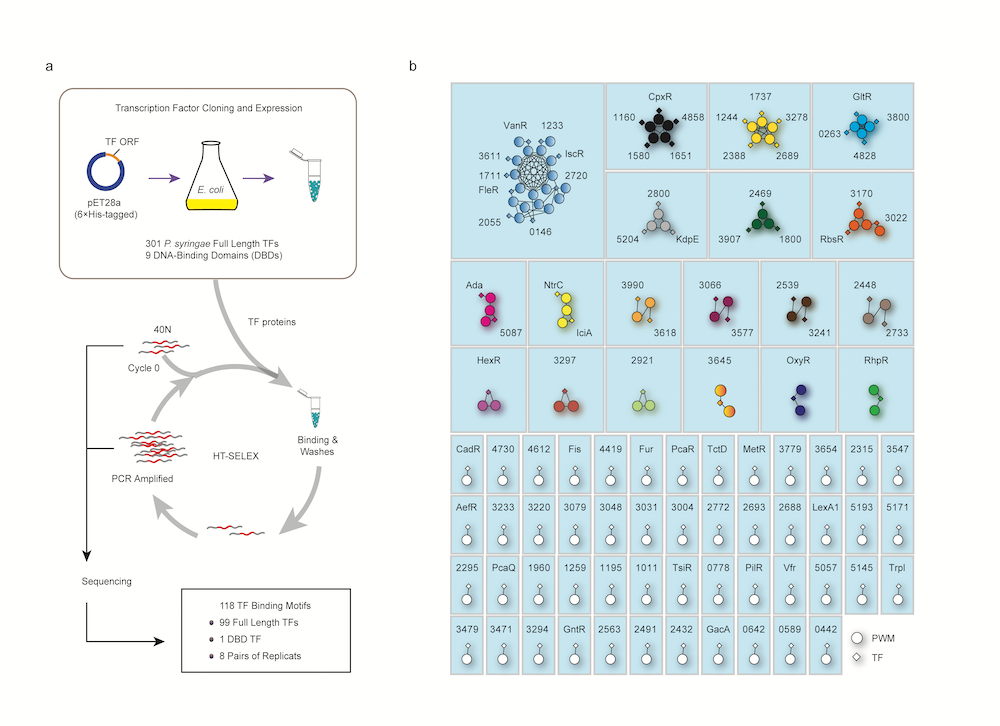
To unveil the regulatory mechanisms of virulence in P. syringae, the research team applied the high-throughput systematic evolution of ligands by exponential enrichment (HT-SELEX) approach on all the 301 TFs for analysis. Established by Dr Yan, HT-SELEX is a high-throughput and quantitative technique used to analyse DNA and proteins’ interaction.
The team analysed the data and revealed the binding motifs of 100 distinct TFs, which would bind with specific DNA sequences. “Interestingly, most TFs exhibit unique binding motifs. We then re-categorised these 100 TFs into 69 different families, according to the similarity of their binding DNA sequences,” added Dr Deng.
The team also projected the genome-wide binding sites of these 100 TFs in plants and their downstream target genes. And the transcription of these genes is controlled by the transcription factors. As a result, the team has outlined the transcriptional regulatory networks of P. syringae, and even discovered some virulence-related regulatory proteins that were not found before.
To further explore the role of TFs in pathogenic mechanisms, the team investigated the transcriptional regulatory networks in the virulence-associated pathways, and successfully identified 25 master regulators of which 14 have not been characterised as TFs before. Master regulators are the transcription factors that appear to control most of the regulatory activities of other transcription factors and the associated genes. Inhibition of these master regulators would help in controlling the virulence of the pathogens. Therefore, master regulators are often good antibiotic targets that can be used for future drug development.
“Our findings this time deepened our understanding of the pathogenic mechanism of P. syringae and provided drug targets in developing drugs to protect crops from being infected by it. The results also provided important information for the future studies on the transcriptional regulation in P. syringae and relevant pathogens,” concluded Dr Deng.

Dr Deng and Dr Yan are both the corresponding authors of the paper. The co-first authors are Dr Wang Tingting and Hua Canfeng, both from BMS at CityU, as well as Dr Fan Ligang and Dr Sun Wenju from the Northwest University. Other CityU team members include postdoc Dr Shao Xiaolong, research assistant Lucas Grunwald, and PhD student Liu Jingui. Other collaborating researchers come from Shanghai Fourth People’s Hospital.


The study received funding support from CityU, the Research Grants Council of Hong Kong SAR, the National Natural Science Foundation of China, China Postdoctoral Science Foundation and Innovation Technology Funds of Hong Kong.
DOI number: 10.1038/s41467-020-18744-7