
Published on nature synthesis (16 January 2023)
Author(s): Xuefeng Tan, Zhiqin Deng, Qingli Wang, Shu Chen, Guangyu Zhu & Jianwei Sun
Abstract
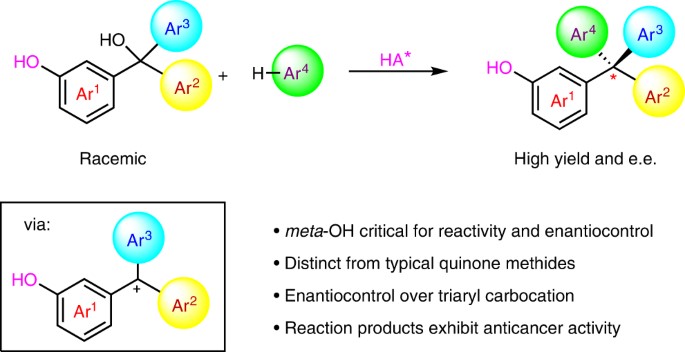
Benzylic carbocations bearing an ortho- or para-hydroxyl group can be stabilized by forming quinone methides, which have been explored in enantioselective synthesis. However, those with a meta-hydroxyl group have remained almost unexplored in organic synthesis. The lack of resonance stabilization by a typical quinone methide form renders them not only difficult to generate, but also challenging to control for asymmetric bond formation. Here we report an efficient catalytic enantioselective reaction between meta-hydroxyl triarylmethanols and indoles, via triaryl carbocations, for the synthesis of tetraarylmethanes with excellent enantiocontrol. Control experiments reveal that the meta-hydroxyl group is essential for both reactivity and stereocontrol. Ortho-directing groups (alkoxyl, sulfenyl or fluoro) benefit enantiocontrol through secondary hydrogen-bonding interactions, but are not required for reactivity. The resulting tetraarylmethane products show anticancer activities, through a mechanism distinct from that of classical anticancer drugs.
Read more: https://www.nature.com/articles/s44160-022-00211-4#Sec8.