
Published on Applied Catalysis B: Environmental (7 May 2022)
Author(s): Guohan Sun, Molly Meng-Jung Li, Keizo Nakagawa, Guangchao Li, Tai-Sing Wu, Yung-KangPeng
Abstract
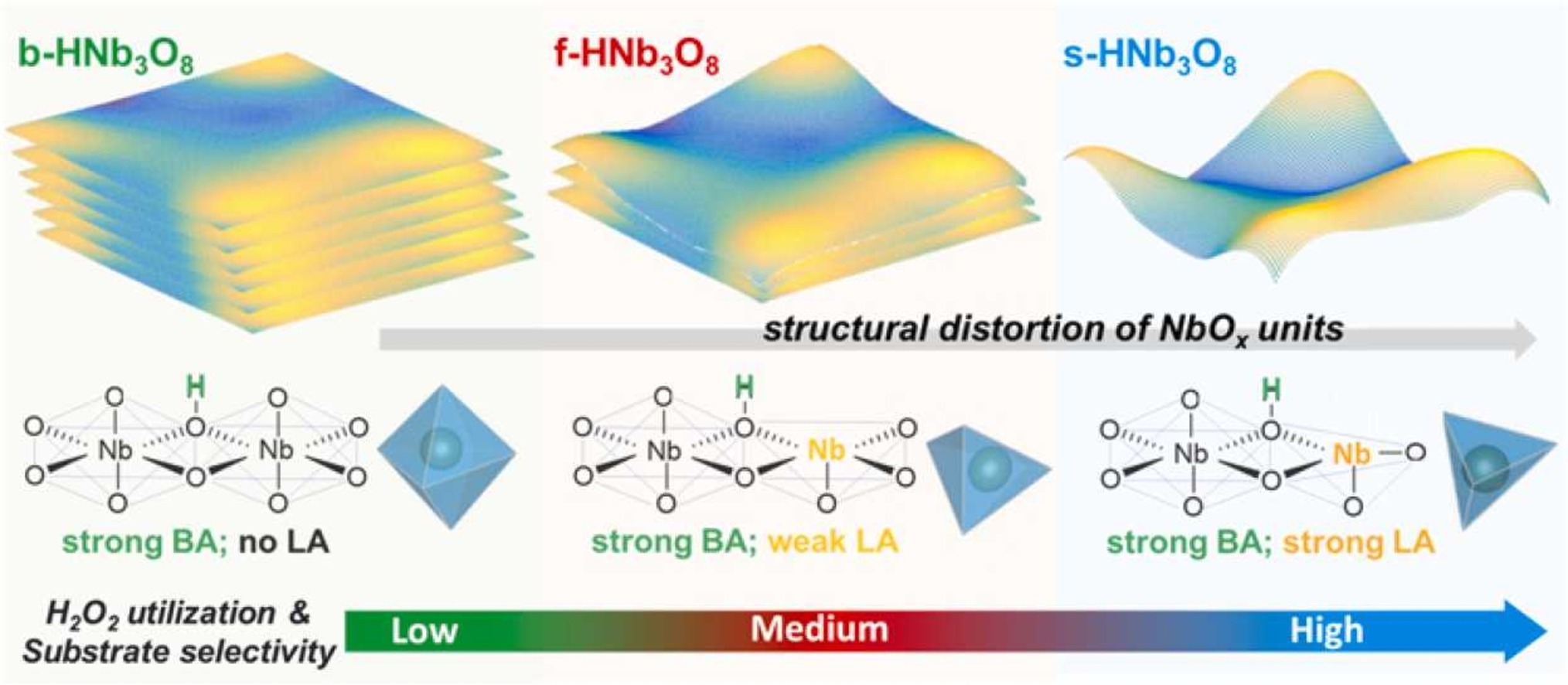
H2O2 has been widely used as a green oxidant in many heterogeneous reactions. However, its disproportionation accelerated by either Brønsted acid (BA) or redox metal sites often results in low H2O2 utilization. Given this, metals less able to redox have been used because the formation of surface metal-peroxo species exhibits a certain degree of selectivity towards substrates. However, those catalysts often bear BA sites on their surface for charge balance, making it imperative to study H2O2 activation pathway in the presence of both sites. Herein, layered HNb3O8 with structurally preserved BA sites was used for this purpose. We found that the exposure of Nb sites via bulk-to-nano regulation of this material hinders H2O2 disproportionation promoted by BA sites. Among various Nb-peroxo species, its bidentate configuration formed on highly distorted NbOx was found to provide the best reactivity in alkene epoxidation with a near stoichiometric H2O2 utilization.
Read more: https://www.sciencedirect.com/science/article/pii/S0926337322004027