Published on Journal of The American Chemical Society (13 October 2021)
Author(s): Xingxing Wei, Taro Matsuyama, Hajime Sato*, Dexiu Yan, Pui Man Chan, Kazunori Miyamoto, Masanobu Uchiyama*, and Yudai Matsuda*
Abstract
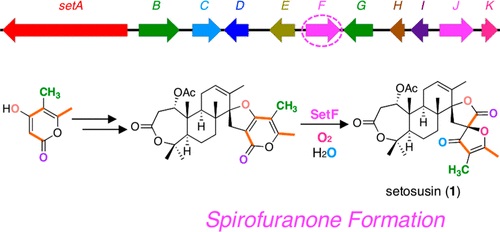
The 3(2H)-furanone unit is observed in many biologically active natural products, as represented by the antifungal medication griseofulvin. Setosusin (1) is a fungal meroditerpenoid featuring a unique spiro-fused 3(2H)-furanone moiety; however, the biosynthetic basis for spirofuranone formation has not been investigated since its isolation. Therefore, in this study we identified the biosynthetic gene cluster of 1 in the fungus Aspergillus duricaulis CBS 481.65 and elucidated its biosynthetic pathway by heterologous reconstitution of related enzyme activities in Aspergillus oryzae. To understand the reaction mechanism to afford spirofuranone, we subsequently performed a series of in vivo and in vitro isotope-incorporation experiments and theoretical calculations. The results indicated that SetF, the cytochrome P450 enzyme that is critical for spirofuranone synthesis, not only performs the epoxidation of the polyketide portion of the substrate but also facilitates the protonation-initiated structural rearrangement to yield 1. Finally, a mutagenesis experiment using SetF identified Lys303 as one of the potential catalytic residues that are important for spirofuranone synthesis.