- 1B-205, 2/F, Block 1, To Yuen Building
- +852 3442-7477
- +852 3442-0549
- Ban.KW@cityu.edu.hk
- CityU Scholars
- Stem Cell Biology • Regenerative Medicine • Cardiovascular Regeneration • Cardiovascular Physiology
Biography
| INSTITUTION | DEGREE | AREA OF STUDY |
|---|---|---|
| Emory University, Atlanta, USA | Instructor | Cardiac stem cell biology |
| Emory University, Atlanta, USA | Post-doc | Cardiac stem cell biology |
| University of Toronto, Toronto, Canada | PhD | Cardiovascular physiology |
| University of Toronto, Toronto, Canada | MSc | Cardiovascular physiology |
| Dongguk University, Seoul, Korea | MSc | Microbiology |
| Dongguk University, Seoul, Korea | BSc | Applied Biology |
Selected Publications
After Joining CityU (#Corresponding Author)
- Choi SW, Shin JS, Park SJ, Jung E, Park YG, Lee J, Kim SJ, Park HJ, Park SM, Moon SH#, Ban K#, Go YY# Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Research. 2020 Dec 01; 18 4: 104955.
- Lee S, Park BW, Lee YJ, Ban K#, and Park HJ#. In vivo combinatory gene therapy synergistically promotes cardiac function and vascular regeneration following myocardial infarction. Journal of Tissue Engineering. 2020 Sep 04; 11: 1–10.
- Park BW, Jung SH, Das S, Lee SM, Park JH, Kim H, Hwang JW, Lee SH, Kim HJ, Kim HY, Jeong S, Cho DW, Jang J#, Ban K#, and Park HJ# ; In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Science Advances. 2020 Mar 25; 6(13).
- Park S, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K#, and Park HJ#; Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nature Communications. 2019 Jul 16;10(1):3123.
- Lee S, Lee DH, Park BW, Kim R, Hoang AD, Woo SK, Xiong W, Lee YJ, Ban K#, and Park HJ# ; In Vivo Transduction of ETV2 Improves Cardiac Function and Induces Vascular Regeneration Following Myocardial Infarction. Experimental & Molecular Medicine. 2019 Feb 12;51(2):13.
- Jiang Y, Park P, Hong SM, Ban K#; Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells: Current Strategies and Limitations. Molecules and Cells. 2018 Jul 31;41(7):613-621.
- Ban K, Bae,SH and Yoon YS; Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics. 2017 May 17;7(7):2067-2077.
Before Joining CityU
- Ban K, Wile B, Cho KW, Kim S, Song MK, Kim SY, Singer J, Syed A, Yu SP, Wagner M, Bao G, and Yoon YS; Non-genetic purification of ventricular cardiomyocytes from differentiating embryonic stem cells through molecular beacons targeting a ventricle-specific transcription factor. Stem cell reports. 2015 Dec 8;5(6):1239-49
- Wile B*, Ban K*, Yoon YS+, and Bao G+; Molecular beacon enabled purification of living cells by targeting cell-type specific mRNAs. Nature Protocols. 2014 Oct; 9(10): 2411-2424 PMID: 25232937 (* and +: contributed equally)
- Ban K, HJ Park, Kim S, K Cho and Yoon YS; Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair in experimental myocardial infarction. ACS Nano. 2014 Oct 28; 8(10):10815-10825 PMCID: PMC4212793.
- Ban K, Wile B, Kim S, Byun J, Saafir T, MK Song, sP Yu, Wagner M, Bao G and Yoon YS; Purification of cardiomyocytes from differentiating human pluripotent stem cells using molecular beacons targeting mRNA of a cardiomyocyte-specific gene. Circulation. 2013 Oct 28: 128(17) 1897-1909 PMCID: PMC3878656.
- Ban K, Kim K, Cho CK, Sauvé M, Diamandis EF, Backx PH, Drucker DJ and Husain M; GLP-1(9-36)-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010 Apr;151(4):1520-31 PMID 20172966.
- Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, Penninger JM, Backx PH, Oudit GY, Tsushima RG; Phosphatidylinositol 3-Kinase gamma is a critical mediator of myocardial ischemic and pharmacological preconditioning. Circulation research. 2008 Sep 12; 103(6): 643-653 PMID 18688045.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M; Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008 May 6; 117(18): 2340-2350 PMID 18427132.
Research Interests
Overview
Heart disease is the leading cause of death in the worldwide with the majority of fatalities due to coronary artery disease and correlating heart failure. Due to limited therapeutic options for severe myocardial infarction and advanced heart failure, stem cell-based therapy has emerged as a promising therapeutic option. Hence, the ultimate goal of our research group is to develop novel therapeutic applications for stem cell based cardiac regeneration using the various types of stem cells including but not limited to the cardiomyocytes and endothelial cells differentiated from human pluripotent stem cells (hPSCs) including both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
In particular, we have focused on studying cardiomyocytes (= Heart muscle cells) derived from (hPSCs) as they are regarded as one of the most promising sources for cardiac repair. Cardiomyocytes differentiated from hPSCs share many similar characteristics with human primary cardiomyocytes. They have unquestioned cardiomyogenic potential, and have a clear cardiac phenotype, cardiac-type electrophysiologic characteristics, and expression of expected genes and proteins. Several recent studies have shown that transplantation of these cardiomyocytes into rodent models helped preserve cardiac function.
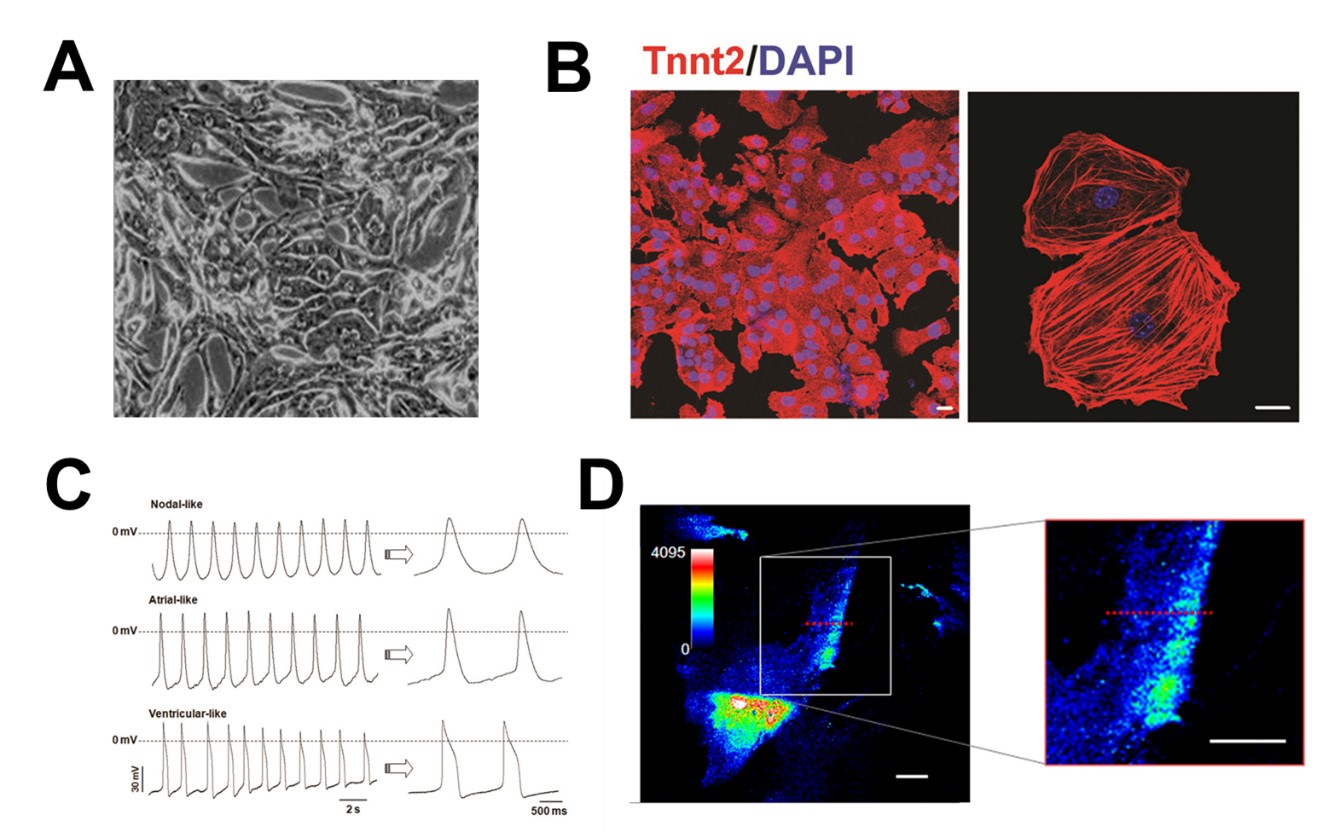
To achieve comprehensive cell based cardiac regeneration, we have been investigating a wide variety of research areas including but not limited to cardiovascular physiology, pathophysiology, pharmacology, tissue engineering, biomaterials, and nanoscience.
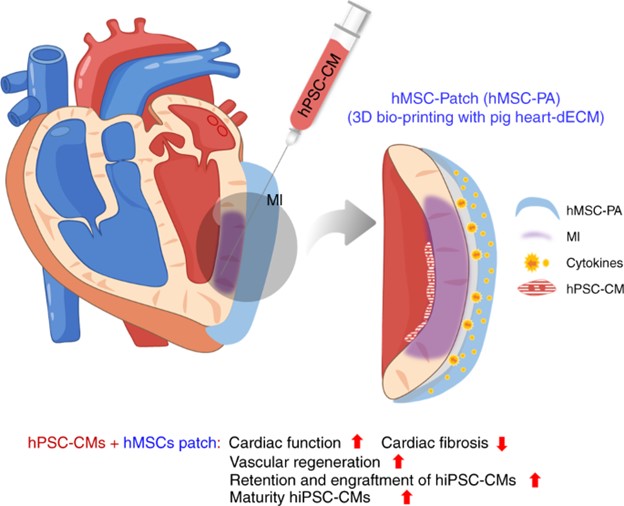
Research News: https://www.cityu.edu.hk/media/news/2019/09/10/first-dual-stem-cell-therapy-brings-new-hope-cardiac-repair
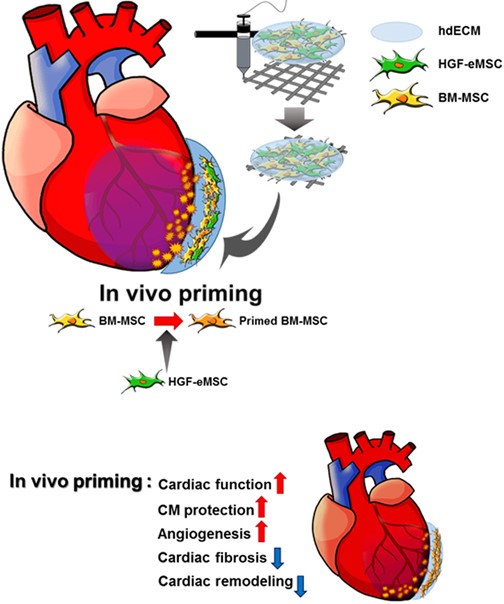
Research Story: https://www.cityu.edu.hk/research/stories/2020/03/30/new-vivo-priming-strategy-train-stem-cells-can-enhance-cardiac-repair-effectiveness
We have ongoing research efforts aimed at:
- Studying the pathophysiological mechanisms of human cardiac disease
- Generation of universal protocol that can efficiently produce large scale of CM derived from human PSCs
- Production of homogeneous population of functionally and structurally mature hPSC-derived cardiomyocytes
- Development of optimal strategies to increase the retention of transplanted human cardiomyocytes in the diseased heart
Available Position(s)
We are currently looking for highly motivated and focused candidates with expertise in cell biology, molecular biology, and physiology. If you are interested in joining our research group, please send your CV to Prof. Ban.
1 July 2022
