Opportunity
Bioorthogonal labeling involves the metabolic or genetic incorporation of a biomolecule of interest containing an abiotic functional group (chemical reporter) into live cells and organisms, and subsequent labeling with a bioorthogonal probe that carries the specific reaction partner. Over the past ~20 years, various chemical reporters and fluorescent bioorthogonal probes have been developed. However, most of these probes are organic dyes that are already fluorescent before the labeling reaction, and hence stringent washouts are required after the staining. Their applications are also limited by high photobleaching rates, substantial self-quenching, high pH dependence, and short-lived fluorescence that is incompatible with fluorescence-lifetime imaging microscopy (FLIM).
Recently, there has been an emerging interest in exploiting new fluorogenic and phosphorogenic bioorthogonal probes which only display emission turn-on after the labeling. Their use eliminates the need for stringent washouts to remove any unreacted probes, enhances the signal-to-noise ratio, and offers the opportunity to monitor biological processes in real time. However, these probes are challenging to construct and should ideally be photostable, not suffer from self-quenching, and exhibit long-lived and pH-independent emission.
Technology
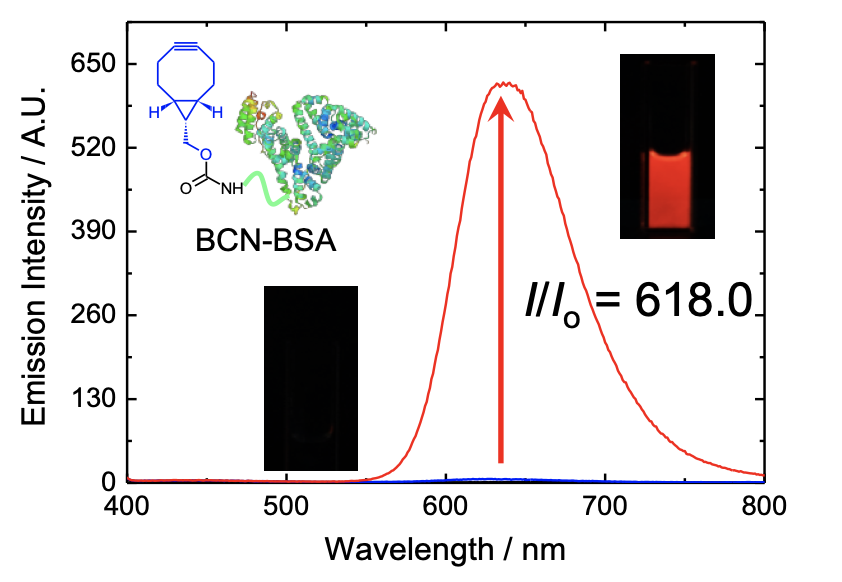
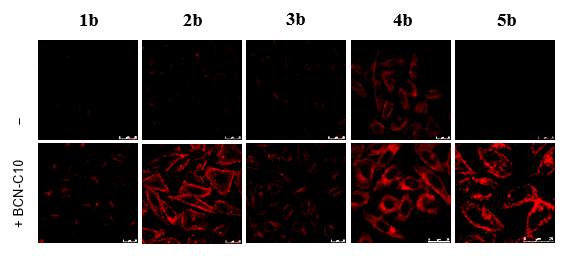
The inventors developed novel transition metal-based phosphorogenic bioorthogonal probes using nitrone as both a bioorthogonal functional group and an emission quencher. The unprecedented use of nitrone was adopted as it: 1) selectively undergoes strain-promoted alkyne–nitrone cycloaddition (SPANC) with a strained alkyne to produce an N-alkylated isoxazoline, and 2) the C=N isomerization provides a facile non-radiative deactivation pathway for fluorescent and phosphorescent compounds. Thus, when the isomerization of the C=N group of nitrone is inhibited after SPANC, the emission quenching pathway disappears, and these compounds are expected to resume their rich emission behavior. The inventors exemplified this idea by incorporating the nitrone unit into luminescent transition metal complexes including iridium(III) and ruthenium(II) polypyridines, and using them as phosphorogenic bioorthogonal probes for biomolecules labeled with a strained alkyne.
Advantages
- Unlike fluorescent organic compounds, most phosphorescent transition metal polypyridine complexes are highly photostable, allowing for continuous tracking and monitoring of the analyte in the stained cells.
- Luminescent transition metal complexes offer unique emission features such as phosphorogenic responses after the bioorthogonal labeling reaction.
- Since phosphorescence originates from a triplet excited state and is associated with a large Stokes’ shift, self-quenching and short emission lifetimes are not issues when using these complexes.
- Their long emission lifetimes can also facilitate the detection and imaging of live cells by FLIM, which offers enhanced sensitivity and lower limits of detection.
- Unlike organic azide dyes, transition metal nitrone complexes are stable and remain intact in the presence of biothiols.
Applications
- To the best of the inventors’ knowledge, all commercially available fluorescent bioorthogonal probes are organic compounds. This invention uses transition metal nitrone complexes as a brand-new alternative.
- The main functions and applications of these complexes are to tag and visualize biomolecules in their native settings (e.g., live cells and animals) via bioorthogonal labeling.
- In the U.S., a large number of biochemical companies, research-oriented institutes, and universities have a strong demand for new biochemicals and reagents that can be applied to a range of research projects.
- New biological reagents will assist researchers working in cellular and animal imaging in chemical biology, biochemistry, and medical science.