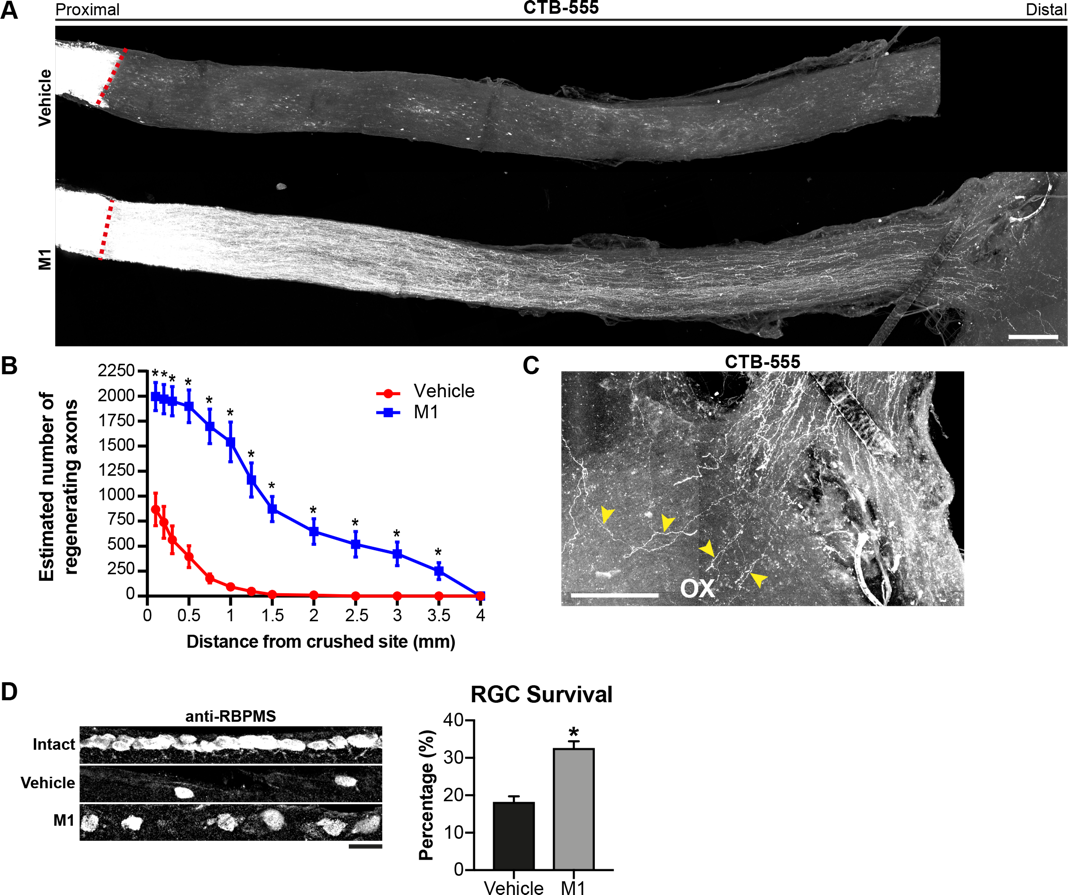
Fig. 1. Mitochondrial fusion promoter M1 induces long-distance axon regeneration 28 days after optic nerve crush injury. 1µg of M1 (1µg/µl) was intravitreally injected into the injured eye at day 0, 7, 14 and 21 after optic nerve crush injury. Three days before termination, 2µg of CTB-555 was intravitreally injected to anterograde label the regenerating axonal fibres. (A and B) M1 treatment induced substantial axonal regrowth 28 days after optic nerve crush injury. Mean ± SEM (n = 5-7 per group). * P<0.05, Student’s t-test. (C) Some of the regenerating axonal fibres (yellow arrowheads) regenerated to the optic chiasm (OX).(D) More RBPMS-positive RGCs were observed in M1-treated mice, compared with vehicle-treated controls. Scale bars: 200µm in (A and C), 20µm in (D).
Opportunity
Nervous system injuries often result in persistent and irreversible sensory and motor function deficits in patients. Proximal peripheral nervous system (PNS) injuries require long-distance axon regeneration for target muscle reinnervation and motor functional recovery. However, adult peripheral neurons have limited intrinsic growth capacity, and the rate of axonal regrowth is slow (i.e., 1–2mm/day). Although the peripheral axons can regenerate into their target muscles, they fail to reform functional synapses at the motor endplate for motor functional recovery, largely due to prolonged muscle denervation. In the central nervous system (CNS), a lack of neuronal intrinsic growth capacity and the presence of extrinsic growth inhibitors are the major obstacles for successful axon regeneration.
Currently, effective drugs to treat patients with CNS/PNS injuries and effective treatment strategies to rekindle the intrinsic growth capacity of injured neurons are lacking. Surgical repair is commonly used for nerve repairs but results in limited functional recovery. Thus, the opportunity exists to revolutionize therapeutic interventions for treating patients with CNS/PNS injuries.
Technology
The inventors identified mitochondrial fusion promoter M1 as a potent small molecule that has the therapeutic potential to enhance the intrinsic growth capacity of adult neurons—an important step as successful axon regeneration after nervous system injuries largely depends on the intrinsic growth capacity of injured neurons. Specifically, they used primary cultures of axotomized dorsal root ganglion (DRG) neurons as an in vitro model to assess the extent of neurite outgrowth after treating the neurons with M1. They found that M1 markedly increases the intrinsic growth capacity of adult DRG neurons in vitro, and accelerates axon regeneration after in vivo peripheral nerve injuries in mice. Remarkably, intravitreal injection of M1 induced robust axonal regrowth, with some of the regenerating axons reaching the superior colliculus and eliciting neuronal firing six weeks after optic nerve crush injury. Thus, this invention proposes M1 as a novel therapeutic strategy for accelerating nerve repair in patients with PNS and CNS injuries.
Advantages
- This is the first report of the therapeutic efficacy of M1 in enhancing axon regeneration after CNS/PNS injury.
- M1 can be applied directly to the crushed site of patients to promote axonal regrowth after peripheral nerve injury.
- Intravitreal injections of M1 on a weekly basis successfully induces substantial axonal regrowth after optic nerve crush injury. At 1 month, some of the regenerating retinal ganglion cell axonal fibers can reach the optic chiasm for target reinnervation to elicit neuronal firing from the superior colliculus.
Applications
- In developed countries, the incidence of peripheral nerve injuries is approximately 13–23 per 100,000 persons annually.
- In the last decade, in China, the occurrence of nerve injuries requiring novel therapies to promote axon regeneration and functional recovery has increased dramatically.
- In the past decade, in the U.S., >US800 million was spent on medical care and rehabilitation for patients with peripheral nerve injuries. Collaboration and the development of drug formulation clinical trials with major manufacturers of M1 in the U.S. would be beneficial if the patent is filed there.
- Local administration of M1 can be used as a treatment for patients with PNS/CNS injuries to promote axon regeneration and functional recovery.