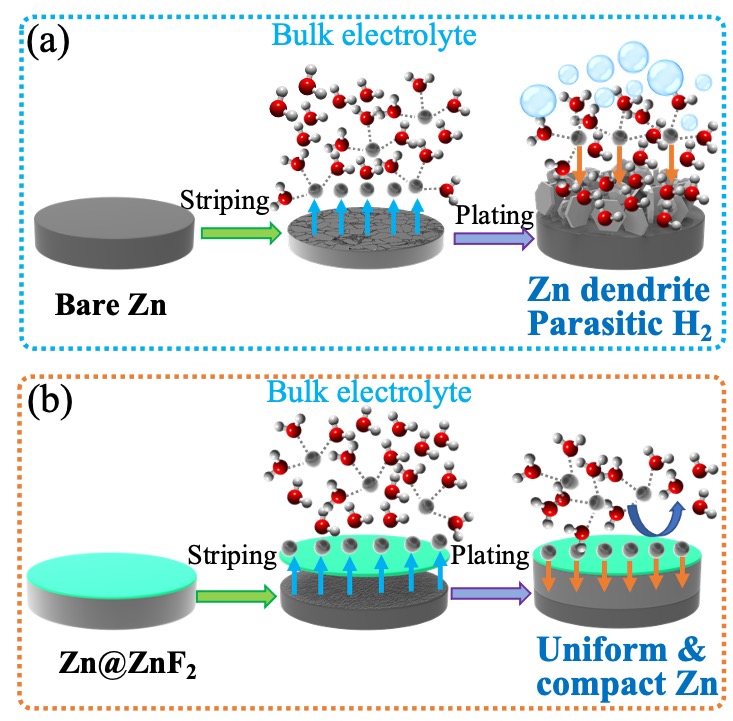
Figure 1(a). Schematic illustration of Zn deposition on bare Zn foil. The corrosion, abundant “dead” Zn, and dendrite are observed due to 2D Zn2+ diffusion and thermodynamically instability of Zn metal in aqueous electrolyte. (b). Schematic illustration of Zn deposition on Zn@ZnF2 foil. The ZnF2 layer endows a dense and dendrite-free Zn deposition by regulating Zn2+ diffusion, controlling nucleation, and prohibiting the permeation of H2O and O2.
Opportunity
With growing demand for electric vehicles, which are more environmentally friendly than fossil fuel-based transportation, there is an increasingly pressing need for efficient and safe rechargeable batteries. Aqueous batteries, which use water-based electrolytes instead of conventional chemical solvents, have been suggested as a safer alternative to rechargeable lithium-ion batteries.
Zinc (Zn) metal anodes are a promising technology for rechargeable aqueous batteries, thanks to their low electrochemical potential and intrinsic safety. However, several problems remain with the application of this technology. The uneven electrodeposition of Zn metal can lead to internal short circuits, leading to safety issues such as the risk of fire, as can the vertical growth of Zn dendrites caused by interaction between Zn and liquid electrolyte.
Strategies to tackle these and other problems with aqueous batteries based on Zn metal anodes, such as electrolyte optimization, are lacking. Due to high costs, a lack of scalability and poor battery performance, these strategies are not yet ready for industrial application.
Technology
The researchers performed quantitative assessment of the hydrogen evolution in Zn metal batteries via in-situ battery gas chromatography mass analysis. Based on this precise assessment, they developed a compact and homogeneous zinc fluoride (ZnF2) solid electrolyte interface (SEI) layer that is formed in situ via a novel vapor-solid method. The SEI layer is not only highly electronically insulating but also highly conductive to zinc cations isolating the zinc metal from the liquid electrolyte. The invention also includes a dendrite-free and side reaction-free Zn anode. Together, this reduces 99.2% of the parasitic hydrogen evolution reaction that occurs during battery cycling and ensures the uniformity of zinc electrodeposition. The battery cell created by the researchers using their ZnF2 layer and Zn anode remained stable over 2,500 cycles, outperforming all reported zinc metal anodes in aqueous systems.
Advantages
- Unlike existing methods, the new method of isolating Zn metal from liquid electrolyte not only reduces more than 99.2% of the parasitic hydrogen evolution reaction during cycling but also guides uniform Zn electrodeposition.
- Outperforms all reported zinc metal anodes in aqueous systems in terms of cycling stability, as well as demonstrating greater safety and stability.
Applications
- Offers inspiration for next-generation aqueous Zn metal batteries with high areal capacity and high reversibility
- Safe, stable alternative to rechargeable lithium-ion batteries for electric vehicle manufacturers