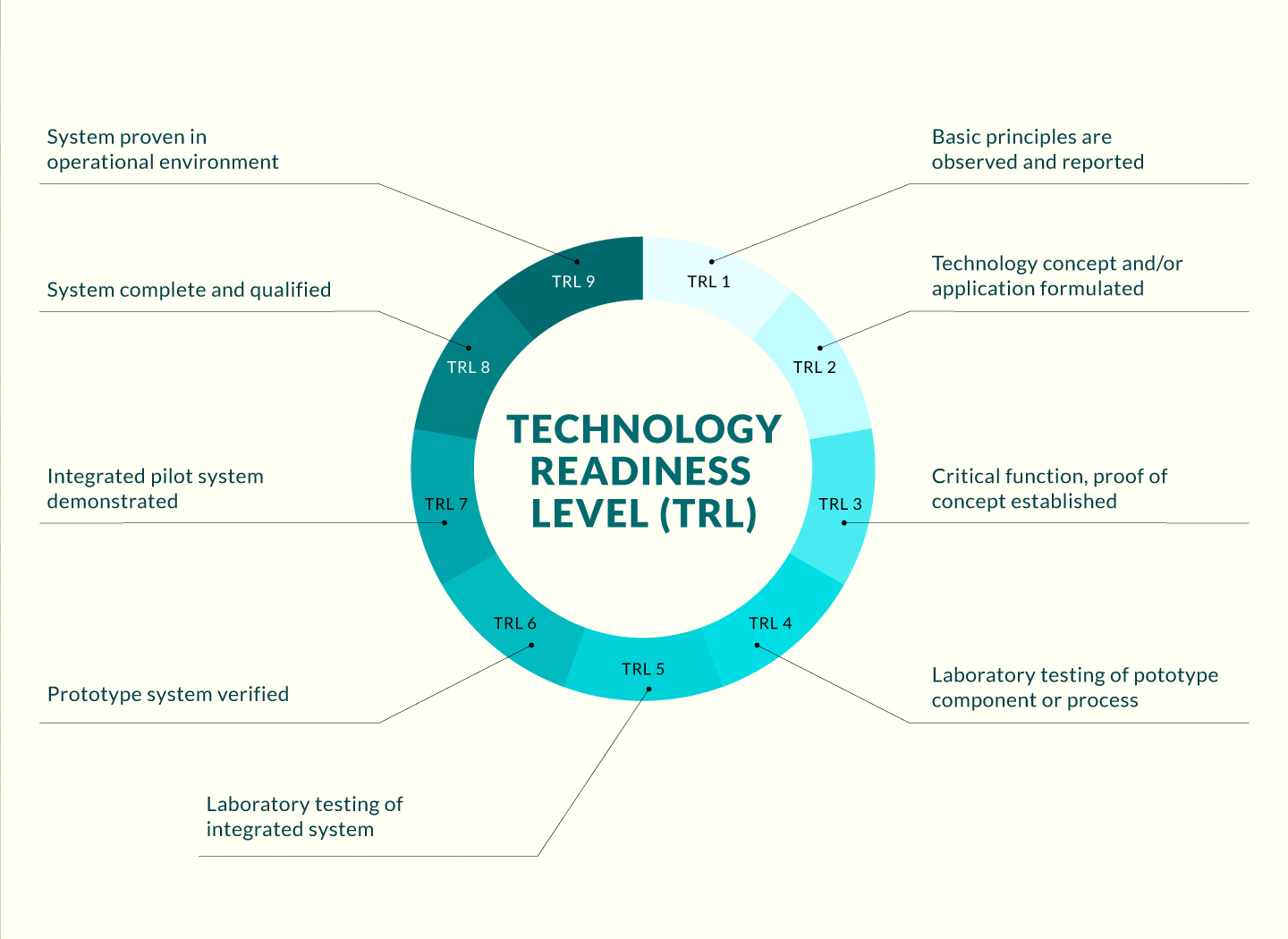
Opportunity
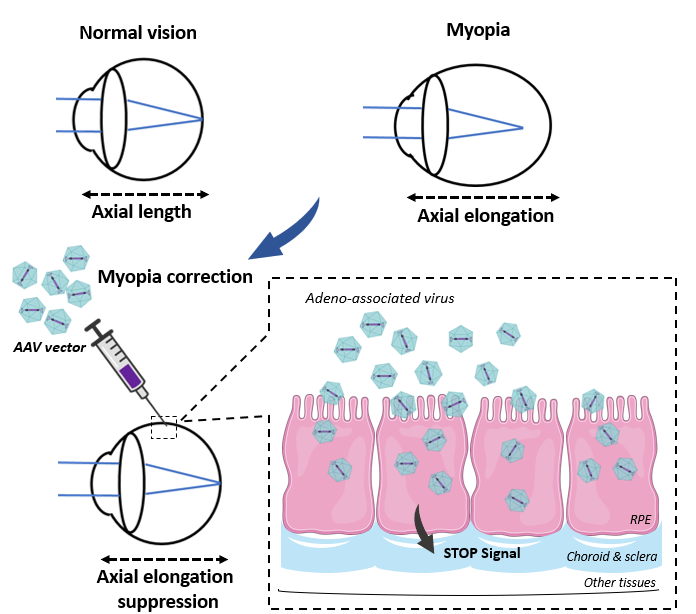
Several human eye diseases are associated with enlarged eye globes. Eye size, especially axial length of the eye globe, is the key parameter of visual function. In the patients with congenital high myopia or buphthalmos that are caused by genetic mutations, their eyes are extremely enlarged with severe refractive errors, leading to compromised vision and secondary ocular pathologies. At present, there is no treatment available for genetic high myopia diseases, such as Bonnai-Barrow and Facio-oculo-acoustico-renal (DB/FOAR) sydrome and Stickler syndrome. Juvenile myopia, which are caused by environmental factors, lifestyle and genetic factors, are also characterized by dysregulated eye growth and increased axial length. Juvenile myopia affect the vision of nearly 50% school-aged children in Hong Kong and other Asian regions according to the newest survey. However, no effective treatment can completely stop the development of myopia. Therefore, there is a huge medical need for an anti-myopic therapy that works for different forms of myopic diseases.
Technology
The inventors developed an in vivo gene therapy to treat congenital high myopia and buphthalmos. This invention was based on their scientific discovery of a novel molecular mechanism controlling animal eye size. In brief, a signaling cascade Srebp2-Lrp2-Bmp2 that initiates in the retinal pigment epithelium (RPE) was found to control eye size. This cascade is composed of both eye growth stop factors (Lrp2 and Bmp2) and eye growth promoting factor (Srebp2). A set of adeno-associated virus (AAV) vectors carrying the RPE-specific promoter sequence and the transgene that change the level or activity of Srebp2-Lrp2-Bmp2 were developed for anti-myopic gene therapy treatment. In animals, intraocular injection of the vectors (such as AAV-best1-Bmp2 and AAV-best1-srebp2 shRNA) prevented the development of the high myopia and associated retinal complications caused by Lrp2 deficiency. Thus these gene therapy vectors may be used to treat human eye diseases caused by Lrp2 mutations as well as to treat juvenile myopia given the shared mechanism of eye size regulation by the signaling pathway.
Advantages
- This is the first treatment proposed for DB/FOAR syndrome or Stickler syndrome.
- This gene therapy is treatment may be safer and is more effective than the existing drugs and treatments for congenital and juvenile myopia., which has side effects.
- This gene therapy targets a non-neuronal cell type, the retinal pigment epithelium, and thus it may be more specific and safer with less side effects.
- Other than gene therapy, small molecule drugs or other inventions that target the components of this eye size pathway can also be applied.
Applications
- This invention can be used to treat patients with DB/FOAR syndrome, Stickler syndrome, or juvenile myopia to save their vision.
- China has a huge population of juvenile myopia patients (~50% of school-aged children), while the U.S. and Europe are seeing an increasing number of myopia patients. DB/FOAR and Stickler syndromes are also found in the U.S. and Europe.