
Published on Angewandte Chemie (27 October 2022)
Author(s): Kaixin Lyu, Shuo-Bin Chen, Eugene Yui-Ching Chow, Haizhou Zhao, Jia-Hao Yuan, Meng Cai, Jiahai Shi, Ting-Fung Chan, Jia-Heng Tan, Chun Kit Kwok
Abstract
RNA G-quadruplex (rG4) structures in the 5’ untranslated region (5’UTR) play crucial roles in fundamental cellular processes. ADAR is an important enzyme that binds to double-strand RNA and accounts for the conversion of Adenosine to Inosine in RNA editing. However, so far there is no report on the formation and regulatory role of rG4 on ADAR expression. Here, we identify and characterize a thermostable rG4 structure within the 5’UTR of the ADAR1 mRNA and demonstrate its formation and inhibitory role on translation in reporter gene and native gene constructs. We reveal rG4-specific helicase DHX36 interacts with this rG4 in vitro and in cells under knockdown and knockout conditions by GTFH (G-quadruplex-triggered fluorogenic hybridization) probes and modulates translation in an rG4-dependent manner. Our results further substantiate the rG4 structure-DHX36 protein interaction in cells and highlight rG4 to be a key player in controlling ADAR1 translation.
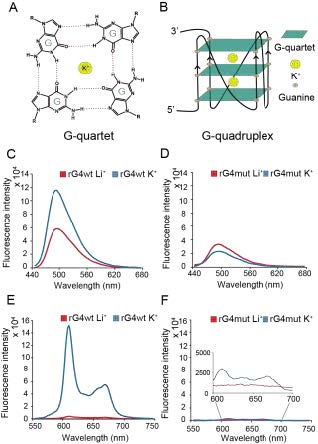
Figure 1. Fluorescence turn-on assay reveals rG4 motif formation in the ADAR5’UTR. (A) G-quartet chemical structure stabilizing by potassium ion (K+) presents in the center. (B) G-quadruplex structure is composed of G-quartets stacking together.(C)(D) ThT ligand-enhanced fluorescence on the (C) ADAR5’UTR rG4wt region and (D) ADAR5’UTR rG4mut region under 150 mM LiCl and KCl conditions, respectively. Spectra under KCl condition in (C) exhibited a positive 2-fold difference in fluorescence (at 494 nm), relative to the LiCl condition, while little difference was observed for (D), suggestingrG4 formation in ADAR5’UTR rG4wt, rather than ADAR5’UTR rG4mut region. (E)(F) NMM ligand-enhanced fluorescence on the (E) ADAR5’UTR rG4wt region and (F) ADAR5’UTR rG4mut region in 150 mM LiCl and KCl, respectively. Spectra under KCl condition in (E) showed a positive 34-fold difference in fluorescence (at 610 nm), relative to the LiCl condition, while only a small difference was observed for (F), indicatingrG4 formation in ADAR5’UTR rG4wt but not in ADAR5’UTR rG4mut region. An inset is shown for the 600-700nm region for (F).
Read more: https://doi.org/10.1002/anie.202203553