Published on Journal of The American Chemical Society (9 September 2021)
Author(s): Huatian Shi, Hung Kay Lee, Yi Pan, Kai-Chung Lau, Shek-Man Yiu, William W.Y. Lam, Wai-Lun Man, Tai-Chu Lau*
Abstract
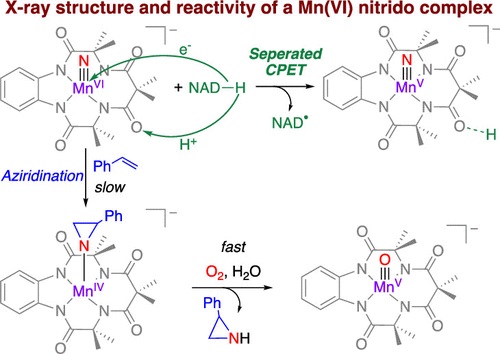
Manganese complexes in +6 oxidation state are rare. Although a number of Mn(VI) nitrido complexes have been generated in solution via one-electron oxidation of the corresponding Mn(V) nitrido species, they are too unstable to isolate. Herein we report the isolation and the X-ray structure of a Mn(VI) nitrido complex, [MnVI(N)(TAML)]– (2), which was obtained by one-electron oxidation of [MnV(N)(TAML)]2– (1). 2 undergoes N atom transfer to PPh3 and styrenes to give Ph3P═NH and aziridines, respectively. A Hammett study for various p-substituted styrenes gives a V-shaped plot; this is rationalized by the ability of 2 to function as either an electrophile or a nucleophile. 2 also undergoes hydride transfer reactions with NADH analogues, such as 10-methyl-9,10-dihydroacridine (AcrH2) and 1-benzyl-1,4-dihydronicotinamide (BNAH). A kinetic isotope effect of 7.3 was obtained when kinetic studies were carried out with AcrH2 and AcrD2. The reaction of 2 with NADH analogues results in the formation of [MnV(N)(TAML-H+)]– (3), which was characterized by ESI/MS, IR spectroscopy, and X-ray crystallography. These results indicate that this reaction occurs via an initial “separated CPET” (separated concerted proton–electron transfer) mechanism; that is, there is a concerted transfer of 1 e– + 1 H+ from AcrH2 (or BNAH) to 2, in which the electron is transferred to the MnVI center, while the proton is transferred to a carbonyl oxygen of TAML rather than to the nitrido ligand.